Early economic modelling and budget impact analysis of Prolaris® test to aid the treatment management decisions in prostate cancer patients
Introduction
To accurately stratify patients with localized prostate cancer according to disease aggressiveness and mortality, Myriad Genetics Inc. developed Prolaris®, a prognostic test that directly measures tumour biology. Although numerous studies indicate that it has prognostic value and may assist in clinical decision-making, the performance and clinical utility have been mainly assessed in the USA.
This research aims to describe the current NHS prostate cancer care pathway in the United Kingdom and determine if the Prolaris® test offers value for money in routine NHS practice.
Data and methods
The data for this project was provided by the Leeds Teaching Hospital Trust, however, due to a significant number of missing values for relevant clinical factors, was later supplemented by Mr William Cross, a consultant urological surgeon in Leeds Prostate Clinic and a co-investigator for this project.
The supplementary information included patient data for nearly 2000 patients who had been referred to the prostate clinic; nearly 50% of whom were subsequently diagnosed with prostate cancer.
Key findings
Prolaris® is intended for use in people with low or intermediate-risk prostate cancer. Based on the Leeds Teaching Hospital Trust data; 18.51% of all prostate cancer patients were classified as belonging to the low and 30.93% to the intermediate D’Amico risk category (D'Amico Risk Classification for Prostate Cancer assesses 5-year risk of treatment failure based on clinical factors, estimates the risk of prostate cancer recurrence (low, medium or high).
Figure 1 shows the most common treatments for low and intermediate risk patients based on Leeds Teaching Hospital Trust data.
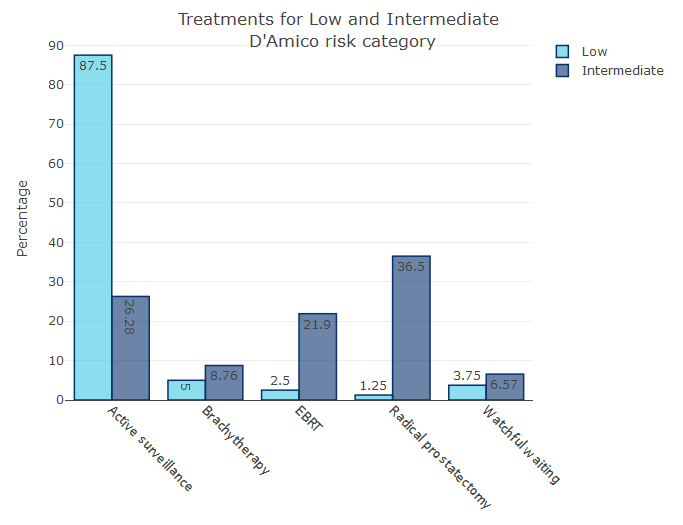
Figure 1- Treatment types in UK: 2014-2017
Various studies refer to the treatment reclassification or disease progression after the 5 or 10 year mark which make it difficult to be applied to this study as patient data is only available for two years. However, based on potentially achievable assumptions of 25% of patients moving from surveillance to treatment before any natural disease progression and at least 20% of patients moving from treatment to surveillance, the application of the Prolaris® test could potentially offer the NHS value for money in this patient group; depending on the price of the test.
The change in treatment recommendation should increase the number of patients appropriately treated but also reduce the uncertainty of disease progression patients’ face whilst being on active surveillance.
Value of the research
This research highlights a need for a better infrastructure for medical data where recording relevant clinical factors is enforced rather than based on the inclinations of the clinician.
The prostate cancer care pathway observed in this study can be used as an overall measure of routine NHS practice in prostate cancer treatment. Furthermore, the cost data obtained during this research can be applied to testing the clinical validity of other prostate cancer drugs and treatments.
Researchers
Cathy Tomson - The University of Leeds
William Cross - The University of Leeds
Dr Gurdeep S Sagoo - The University of Leeds
